Scientific Calendar March 2019
How can fluorescence flow cytometry technology in XN-Series help to differentiate reactive monocytosis from chronic myelomonocytic leukaemia (CMML) samples?
By using the dispersion parameter MO-WX in combination with the NEUT/MONO ratio and MONO#, which was published as ‘Mono-dysplasia score’.
By using the dispersion parameter NE-WX in combination with NEUT/MONO ratio and MONO#, which was published as ‘Mono-dysplasia score’.
No help is needed here. CMML always has MONO# > 3.0 x 10^9/L.
By using the dispersion parameter MO-WX alone.
Congratulations!
That's the correct answer!
Sorry! That´s not completely correct!
Please try again
Sorry! That's not the correct answer!
Please try again
Notice
Please select at least one answer
Scientific background information
Chronic myelomonocytic leukaemia (CMML)
Chronic myelomonocytic leukaemia (CMML) is a malignancy of the blood-forming cells in bone marrow. It is a highly variable condition very similar to different types of bone marrow and blood cancers with a clonal disorder of monocytes. The WHO classifies the disease as myelodysplastic/myeloproliferative neoplasms (MDS/MPN). In healthy individuals, monocytes represent less than 1.0 x 109/L and 10% of the leucocytes, respectively. In CMML, too many stem cells develop into monocytes, but not all of them become mature ones. As a result, the peripheral blood shows monocytosis and dysplastic forms of neutrophils and monocytes, which are typical for CMML, but dysplasia can also be seen in red blood cells and/or platelets. In up to 80% of CMML cases blast cells are absent, which makes diagnosis difficult in the early stages of CMML.
CMML is a rare malignancy with a median age at diagnosis ranging from 65 – 75 years. There are approximately twice as many male patients as female ones. The signs and symptoms can vary and often may include weakness and fatigue due to anaemia, petechiae, bruising and bleeding caused by thrombocytopenia, infections and enlargement of the spleen and liver.
Patients who are finally diagnosed with CMML may have sought a health check at first because of physical weakness, infection or unexplained bleeding. Usually, a diagnosis of CMML cannot be easily confirmed. The WHO diagnosis criteria are persistent monocytosis greater than or equal to 1.0 x 109/L with monocytes accounting for more than 10% of the WBC, dysplasia affecting at least one lineage in blood or bone marrow, blasts in bone marrow and/or in blood less than 20% and/or presence of a clonal abnormality. Diagnosis of CMML requires a thorough blood smear and bone marrow examination as well as cytogenetic, immunophenotyping and molecular analyses (1).
CMML is a very heterogeneous disease and its latent forms may be overlooked. However, early diagnosis of CMML is necessary to prevent serious consequences such as infections and haemorrhagic complications, and to delay the transformation into acute myeloid leukaemia. For some patients with favourable prognostic factors, observation without treatment may be appropriate. For others, supportive care may be provided; hypomethylating drugs can reduce myelodysplastic-like features, and cytoreductive agents are used to control myeloproliferative forms (2).
‘Mono-dysplasia score’
In daily practice, laboratories often have to deal with samples presenting increased monocyte counts. The current method of further inspecting such samples is a blood smear review, depending on certain criteria. In their criteria, both the International Society for Laboratory Hematology and the French-speaking Group for Cellular Haematology consider MONO ≥ 1.5 x 109/L as the cut-off for a monocytosis smear review (3, 4). Since the WHO defines monocytosis in CMML samples as MONO ≥ 1.0 x 109/L and ≥ 10% of the white blood cell count, some CMML cases will not be detected by the aforementioned cut-off. However, when monocytosis is found, it is of a reactive aetiology in the vast majority of cases. In the case of isolated elevated monocyte counts of reactive origin, the smear review does not add any useful information (5).
A new workflow concept is offered with the Extended IPU, which allows following the more stringent WHO criteria for CMML detection (1) and supports in selecting the right monocytosis samples for smear revision. This concept is primarily based on a ‘Mono-dysplasia score’, which was evaluated and published by Schillinger F et al. in 2018 (6). The goal of this approach is to optimise the handling of samples flagged for monocytosis and thereby reduce smear reviews, while increasing the sensitivity of CMML detection for further inspection.
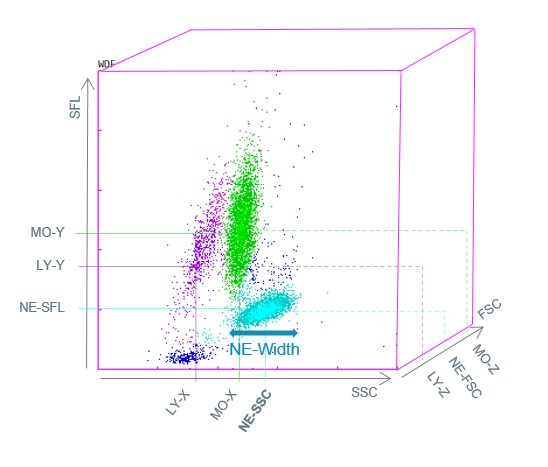
The Sysmex XN-Series analysers use fluorescence flow cytometry in the WDF measurement channel to differentiate the white blood cells into their subpopulations. After the reagent reaction, the cells flow through a laser beam and three measurement signals are detected for each cell simultaneously – forward scattered light (FSC), side scattered light (SSC) and side fluorescence light (SFL). This allows not only the assessment of morphological features, but also cell functionality.
The ‘Mono-dysplasia score’ is based on data from the WDF measurement channel such as monocyte and neutrophil counts and the structural neutrophil dispersion (NE-WX) parameter. NE-WX reflects polymorphonuclear neutrophil structural dispersion, since neutrophil dysplasia is the most frequently observed morphological abnormality.
The NE-WX is based on the side scatter width of the neutrophil population (NE-Width) in relation to its median side scatter position (NE-SSC) (Fig. 1). It is a highly sensitive parameter for identifying the coexistence of minimally granular neutrophils and normal neutrophils, particularly when morphological abnormalities are difficult to identify by microscopic examination. The combination of NE-WX, NEUT/MONO ratio and MONO# parameters into a single ‘Mono-dysplasia score’ was found to be a new successful approach in distinguishing CMML from reactive monocytosis. The positivity of the ‘Mono-dysplasia score’ guided microscopic examination in search of CMML abnormalities with a sensitivity of 96.7% and a specificity of 97.8%, and improved the detection of CMML while decreasing the number of useless blood smear reviews performed for monocytosis in adults (6).
Scattergram
References
(1) Arber DA et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016; 127:2391-2405.
(2) Patnaik MM et al. Chronic Myelomonocytic Leukemia: Focus on Clinical Practice. Mayo Clin Proc. 2016 Feb; 91(2):259-72.
(3) http://www.islh.org/web/consensus_rules.php
(4) Genevieve F et al. Smear microscopy revision: propositions by the GFHC. Feuillets de Biologie 2014; VOL LVI N° 317.
(5) Cornet E et al. Evaluation and optimization of the extended information process unit (E-IPU) validation module integrating the Sysmex flag systems and the recommendations of the French-speaking cellular hematology group (GFHC). Scand J Clin Lab Invest. 2016; 76(6):465.
(6) Schillinger F et al. A new approach for diagnosing chronic myelomonocytic leukemia using structural parameters of Sysmex XNTM analyzers in routine laboratory practice. Scand J Clin Lab Invest. 2018 May; 78(3):159-164.